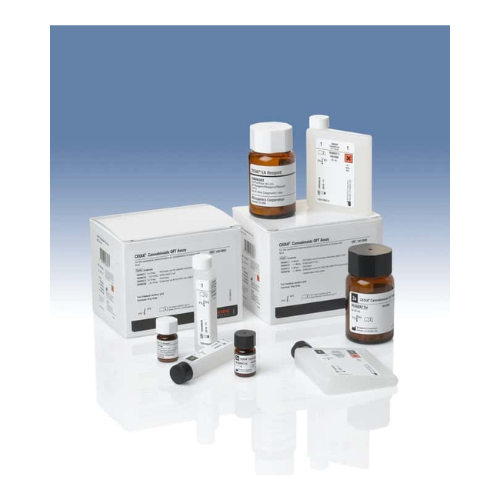
For Business Use Only. Does Not Ship to Residential Addresses. For use inside an Analyzer, Sold Separately.
Thermo Kit EtG Rgt DRI CJF
In Stock
Product code: T-10016154
MPN: 10016154
Manufacturer: Thermo Scientific
Shipping Weight: 2.00lbs (0.91kg)

Meet your sales rep Lee Doughton, for the West USA region.
Would you like to schedule a 30-minute consultation with Lee Doughton?
Yes, Schedule a callThermo Kit EtG Rgt DRI CJF
Specifications
- Brand: DRI®
- Manufacturer: Ortho Clinical Diagnostics
- Country of Origin: United States
- Application: Reagent
- For Use With: For Clinical Chemistry Analyzers
- Form: Liquid
- Sample Type: Urine Sample
- Storage Requirements: Requires Refrigeration
- Test Name: Ethyl Glucuronide (EtG)
- Test Type: Drugs of Abuse
- Volume: 3x18 mL
Intended Use
The Thermo ScientificTM DRI Ethyl Glucuronide Enzyme Immunoassay is intended for the qualitative and semi-quantitative determination of Ethyl Glucuronide in human urine at cutoffs of 500 ng/mL and 1000 ng/mL.
Summary and Explanation of the Test
Ethyl Glucuronide (EtG) is a direct metabolite of ethanol, which is formed by enzymatic conjugation of ethanol with glucuronic acid. Alcohol in urine is normally detected for only a few hours, where as EtG can be detected up to several days even after complete elimination of alcohol from the body. Currently EtG is monitored by GC/MS and LC/MS/MS.
The DRI® Ethyl Glucuronide Assay is supplied as a liquid ready-to-use homogeneous enzyme immunoassay. The assay uses specifi c antibodies that can detect Ethyl Glucuronide without any significant cross-reactivity to other glucuronide compounds. The assay is based on competition between a drug labeled with glucose-6-phosphate dehydrogenase (G6PDH), and free drug from the urine sample for a fi xed amount of specifi c antibody binding sites. In the absence of free drug from the sample, the specifi c antibody binds the drug labeled with G6PDH and causes a decrease in enzyme activity. This phenomenon creates a direct relationship between the drug concentration in urine and enzyme activity. Active enzyme converts NAD to NADH resulting in an absorbance change that can be measured spectrophotometrically at 340 nm.