Kim Huynh, Guohong Wang, Rehka Barhate, Cynthia Coulter, Christine Moore, Warren Rodrigues, Philip Catbagan, Michael Vincent, James Soares, Immunalysis Corporation, Pomona, CA USA
Abstract
Zolpidem (Ambien®) is a non-benzodiazepine sedative-hypnotic drug used for treatment of insomnia. The drug is available as either normal release tablets (5-10mg tablets ) or sustained release tablets (6.25-12.5mg)[1] . From 2001 -2006, the prescription rate of zolpidem in the United States increased from 18 to 28 million[2]. This increase in popularity has resulted in an increase in the number of individuals driving while under the influence of zolpidem [3].
For this reason, a homogeneous enzyme immunoassay (HEIA) to detect zolpidem in urine has been developed. The antibody is highly specific for zolpidem; it does not cross react with other sleep aids such as zopiclone, or other commonly prescribed drugs. The assay has a detection limit of 5 ng/mL.
Objectives
- To develop a high throughput HEIA for the detection of zolpidem in human urine
- o ensure the agreement between immunoassay and LC-MS/MS is >95%
- To ensure minimal interference from commonly used drugs
Methods
The HEIA uses a zolpidem antibody and zolpidem labeled enzyme (glucose-6-phosphate dehydrogenase, G6PDH). The assay is based on the competition between zolpidem labeled G6PDH and the free drug in the urine sample for the fixed amount of antibody binding sites. In the absence of the free drug, the antibody binds the drug enzyme conjugate and enzyme activity is inhibited. Free drug in urine reverses the inhibition. This creates a dose response relationship between drug concentration in the urine and enzyme activity. The enzyme G6PDH activity is determined at 340nm spectrophotometrically by the conversion of NAD to NADH.
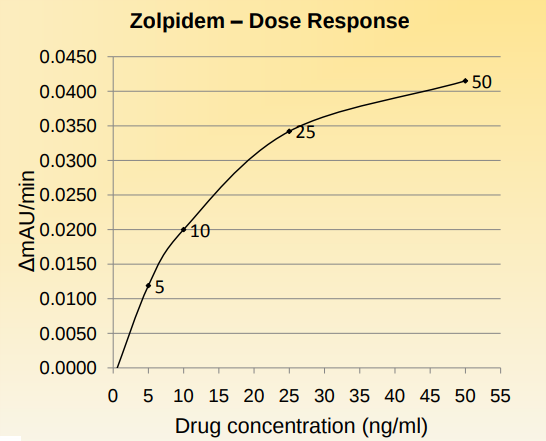
Results and Discussion
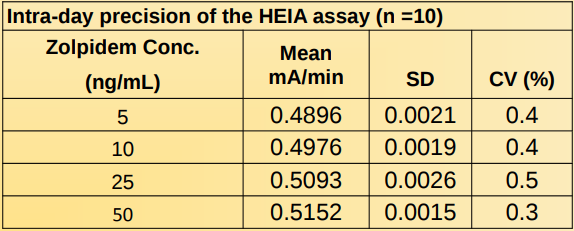
Intra-assay Precision:
Precision studies were determined by assaying calibrators and controls using 10 replicates.
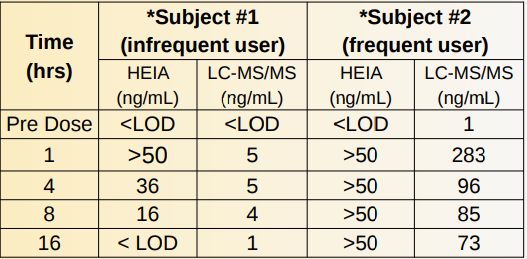
Time Dependent Dosage Study:
A time dependent dosage study was performed using volunteers to evaluate the efficacy of the HEIA. Quantitation using LC-MS/MS was also performed to determine the accuracy of the assay.
Prior to the ingestion of a 10 mg dose of zolpidem (Ambien ®) the subjects provided initial pre-dose urines. Sample collections post-dose were taken at intervals of 1, 4, 8, and 16 hrs. For the HEIA, all urine specimens were tested directly against the calibration curve with no pre-treatment or dilution . The enzyme HEIA assay was able to detect zolpidem in urine for at least 8 hours after ingestion of a 10 mg dose.
In subject#1, the HEIA yielded a higher concentration than LC-MS/MS which may be ascribed to the cross reactivity of the HEIA assay toward other zolpidem metabolites.
Summary
A high throughput and reliable HEIA has been developed for the detection of zolpidem in human urine.
References
- R.C. Baselt and R.H. Cravey. Disposition of Toxic Drugs and Chemicals in Man, 8 th edition. 1673-1675.
- L. Reidy, W. Genero, B.W. Steele, and H.C. Walls. The incidence of zolpidem use in suspected DUI drivers in Miami-Dade Florida; a comparative study using Immunalysis zolpidem ELISA kit and GC-MS screening. J.Anal. Toxicol. (2008) 32: 688-694.
- B.K. Logan and F.J. Couper. Zolpidem and driving impairment. J. Forensic Sci. (2001) 46: 105-110.
SOFT, Oklahoma City, 2009
To view the full study and its data, visit this link.
Looking for equipment or accessories for your homogeneous immunoassay applications? Check out our featured products below.


