Roche Cobas e411 immunoassay analyzer
In Stock
Product code: Roche-Cobas-E411
Manufacturer: Hitachi/Roche
Shipping Weight: 948.00lbs (430.01kg)

Meet your sales rep Lee Doughton, for the West USA region.
Would you like to schedule a 30-minute consultation with Lee Doughton?
Yes, Schedule a call

Free, Simple Tactics to Lower Your Cost Per Test
Get the free guide that shows exactly how to reduce your cost per test using 35 proven tactics, or use the free, fast cost-per-test reduction planner to build a simple, personalized action plan with tactics your lab can implement to reduce wasted budget this year.
Free, Simple Tactics to Lower Your Cost Per Test
Get the free guide that shows exactly how to reduce your cost per test using 35 proven tactics, or use the free, fast cost-per-test reduction planner to build a simple, personalized action plan with tactics your lab can implement to reduce wasted budget this year.

Get the fastest STAT turnaround in its class for up to 60% less than new. Because your ER patients and your budget both deserve better.
The Roche Cobas e411 delivers 86 tests per hour with something no competitor can match: 9-minute STAT results for cardiac markers. When a chest pain patient needs troponin, you're not waiting 30-45 minutes like you would on other benchtop systems. You're making decisions while the patient is still in triage.
This analyzer runs the same Elecsys reagents as Roche's high-throughput reference lab systems. Your results correlate perfectly with the big hospitals. Your price tag doesn't have to.

Why labs choose refurbished Cobas e411 analyzers from Block Scientific:
Cut equipment costs by up to 60%
Same Roche quality, fraction of the investment.
9-minute cardiac STAT
Fastest in class for troponin and other critical markers.
Reagents at fair prices
Lower your cost per test with our Elecsys pricing.
Service that costs up to 50% less
Local techs who know these systems inside and out.
Ships in days, not months
From our US inventory while others quote 16-week lead times.
Trade in your old immunoassay analyzer
Your Mini Vidas or Access has value.
90-day to 1-year warranty
Full protection on every refurbished system.
Cobas e411 specifications that matter
The e411 uses Roche's electrochemiluminescence (ECL) technology - the same detection method found in their million-dollar reference lab platforms. Over 20,000 units installed worldwide since 2006. That's not new technology finding its feet. That's proven performance you can count on.
Core performance metrics
What sets the AU5810 apart for independent and international labs

9-minute STAT changes emergency medicine.
Other benchtop immunoassay analyzers deliver first results in 30-45 minutes. The e411 does it in 9. For high-sensitivity Troponin T, that's not a spec sheet detail - it's the difference between treating a patient and waiting for information. Your ER physicians will notice.

2-month calibration stability saves real money.
Most immunoassay systems need calibration every 1-4 weeks. The e411 holds calibration for 8+ weeks on most assays. That's fewer calibrator purchases, less QC material consumption, and less tech time babysitting the analyzer. The savings add up quietly but consistently.

Elecsys reagents mean universal correlation.
Running the same assays as the reference lab down the street? Your results will match. The e411 uses identical reagent packs to Roche's high-throughput e601, e801, and integrated Cobas systems. No methodology differences to explain away.
Cobas e411 test menu covers what generates revenue
The e411 supports 100+ Elecsys immunoassays. Here's what actually matters for most labs:
Thyroid function (the bread and butter)
TSH (third-generation), Free T4, Free T3, Total T4, Total T3, T-Uptake, Thyroglobulin, anti-TPO, anti-Tg, anti-TSH receptor antibodies
Cardiac markers (where speed matters most)
High-sensitivity Troponin T (the gold standard with 9-minute STAT), NT-proBNP for heart failure, CK-MB mass, Myoglobin, D-Dimer
Tumor markers
PSA (total and free), CEA, AFP, CA 125 II, CA 19-9, CA 15-3, CA 72-4, Cyfra 21-1, NSE, HE4. The HE4 + CA 125 combination enables the ROMA algorithm for ovarian cancer risk assessment - FDA-cleared and clinically meaningful.
Fertility and reproductive hormones
FSH, LH, Estradiol, Progesterone, Prolactin, Testosterone, AMH (Anti-Müllerian Hormone - Roche was first to market with FDA clearance), total hCG, Free β-hCG, PAPP-A for prenatal screening
Infectious disease
HIV combo (4th gen), complete Hepatitis B and C panels, Syphilis, TORCH panel (CMV, Toxo, Rubella with IgG/IgM/avidity), HSV-1 and HSV-2
Everything else
Ferritin, B12, Folate, PTH, Vitamin D, Cortisol, Insulin, C-peptide, Procalcitonin, IL-6
Elecsys reagents at prices that protect your margins
The Cobas e411 uses a closed reagent system. That means Elecsys packs only - no third-party alternatives exist for the immunoassay side. But it also means consistent results, no lot-to-lot variability headaches, and perfect correlation with other Roche platforms.
Block Scientific supplies Elecsys reagents at prices that make the closed system easier to stomach.

What you get with Elecsys reagents
We level the playing field. Block Scientific stocks the complete OSR reagent line at prices that let independent labs compete:
Ready to use - No reconstitution, no mixing, no preparation. Load and run.
60-day onboard stability - Less waste from expired reagents sitting in the cooler.
Barcode programming - The analyzer reads everything it needs from the pack. No manual data entry errors.
100 tests per pack - Standard sizing across the menu.
Standing orders keep you running. Tell us your monthly volume and we'll make sure you never run out. Predictable costs, no emergency shipping charges, no scrambling when Friday afternoon reveals you're low on TSH.
Cobas e411 service that actually responds
Roche service is good when it shows up. The problem is waiting. And paying. Block Scientific service costs up to 50% less than OEM contracts and we're not bound by territory restrictions.
Block Scientific e411 service delivers
Response in 24-48 hours
Not "we'll get back to you next week"

Not generalists learning on your equipment

Same quality, different price

The e411 is reliable when maintained properly
What e411 maintenance actually involves
Daily (5 minutes)
Clean probes, check waste levels, empty solid waste tray.
Weekly
Clean rinse stations, run SysClean cycle.
Annually
Full PM including probe replacement, tubing, seals, and filters.
The e411 isn't high-maintenance. Most "problems" are actually dirty probes or expired reagents. Our service techs can often walk you through fixes over the phone.
Cobas e411 vs the competition - honest comparisons

Cobas e411 vs Beckman Access 2
The Access 2 is the industry's reliability champion. Twenty years of operation is common. It's the Toyota Camry of immunoassay - utterly dependable and any competent tech can service it.
But the e411 wins on speed. STAT results in 9 minutes versus 30-45 minutes on the Access 2. If cardiac markers drive your immunoassay volume, that gap matters. The e411 also holds calibration longer (2 months vs 4-8 weeks) and maintains reagent stability longer onboard (60 days vs 28-56 days).
Choose the Access 2 if:
Maximum reliability and serviceability are your priorities, you don't need rapid cardiac STAT, or you want the most field-proven platform available.
Choose the Access 2 if:
Maximum reliability and serviceability are your priorities, you don't need rapid cardiac STAT, or you want the most field-proven platform available.

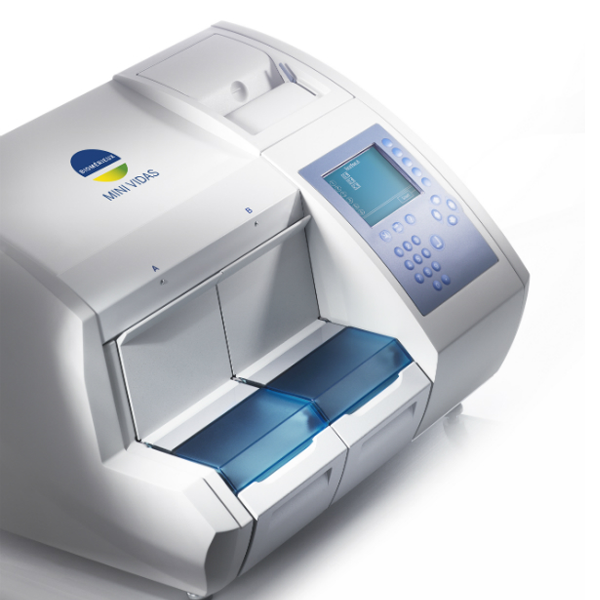
Cobas e411 vs bioMerieux Mini Vidas
The Mini Vidas is tiny (fits almost anywhere) and bulletproof reliable. But it processes samples sequentially - one at a time per strip position. Throughput maxes out around 36 tests per hour.
The e411 delivers 86 tests per hour with true random access. For labs running 50+ immunoassays daily, that throughput difference is the difference between keeping up and falling behind.
Choose the Mini Vidas if:
You run very low volumes (under 30 tests daily), space is severely constrained, or you need specific VIDAS-only assays.
Choose the e411 if:
You need real throughput for routine immunoassay volume.

Cobas e411 vs Siemens Immulite 2000
The Immulite 2000 offers 200 tests per hour and something the e411 can't match: 350+ allergen-specific IgE assays and dedicated veterinary panels. If allergy testing is part of your menu, the Immulite is your only real option in this class.
But the e411 wins on STAT speed (9 minutes vs 15-42 minutes to first result) and footprint (benchtop vs floor model). It's also simpler to operate and maintain.
Choose the Immulite if:
You need allergy testing or dedicated veterinary assays.
Choose the e411 if:
You don't need allergens and want faster STAT in a smaller package.
Which labs benefit most from the Cobas e411?

Small community hospitals (25-100 beds)
Your ER needs cardiac markers fast. Your budget can't justify a floor-standing system. The e411 delivers 9-minute troponin in a benchtop footprint at a refurbished price point that makes financial sense.

Urgent care centers with lab services
Same story, smaller scale. Chest pain patients need rapid triage. The e411's cardiac panel - Troponin T, CK-MB, myoglobin, NT-proBNP - runs in minutes, not the hour-plus turnaround you'd get from a reference lab send-out.

Specialty practices bringing testing in-house
Endocrinology clinics running thyroid panels. Cardiology practices monitoring heart failure with NT-proBNP. Oncology offices tracking tumor markers. Fertility clinics running AMH and reproductive hormones. The e411's menu covers these specialties comprehensively.

Reference labs needing backup capacity
Your primary high-throughput system goes down for maintenance. Do you stop testing or switch to backup? The e411 uses identical Elecsys reagents - same packs, same results, no separate inventory. When your e801 needs service, the e411 keeps you running.

International facilities and developing markets
The e411's global installed base means parts availability, service knowledge, and reagent supply are established worldwide. At refurbished pricing, it brings reference-lab-quality immunoassay to markets where new equipment costs are prohibitive.
Why buy refurbished Access 2 immunoassay systems
The math makes sense
Refurbished e411 analyzers run up to 60% less than new. That's capital you can redirect to reagents, staffing, or other equipment needs. The analyzer delivers identical results either way.
Technology that's proven, not experimental
The e411 has been in production since 2006. Every software bug is patched. Every mechanical weakness is documented. Every common failure mode has a known fix. You're buying 19 years of field experience, not beta testing someone's latest release.
Roche still fully supports it
Elecsys reagents, replacement parts, software updates - all still available and actively manufactured. The e411 isn't an orphaned platform. It's a current product with long-term support.
Expected lifespan of 10-15+ years
These analyzers last. The ECL detection system and fluid handling components are robust. With proper maintenance, a refurbished e411 has years of productive service ahead. Many labs run these systems well past the 15-year mark.
Sell us your immunoassay analyzer
Upgrading to something with more throughput? Consolidating platforms? Your old immunoassay system has value even if it's not running perfectly.
We buy all major platforms





Cobas e411, e601, Elecsys series
Access, Access 2, DxI series
Mini Vidas, Vidas, Vidas 3
Architect i1000, i2000, AxSYM
Centaur, Immulite series
Your old analyzer helps someone else
That Mini Vidas you're replacing? It's exactly what a startup lab in another country needs. Your non-functional Immulite? Its parts keep other labs running. Even equipment beyond repair has value for training programs.
We handle everything: evaluation, decontamination, removal, and logistics. Your trade-in credit applies directly to your e411 purchase.
Comparing Cobas e-series models - when does the e411 make sense?
The e411 is Roche's only standalone benchtop immunoassay option. Everything else requires platform infrastructure. If you need more than 150 samples daily, you're looking at modular systems with substantially higher costs and space requirements.
All Cobas e-series systems use identical Elecsys reagents. Results correlate perfectly across platforms.
Frequently asked questions about the Roche Cobas e411
Figure 100-150 samples in a standard 8-hour shift with typical test menus. The 86 tests/hour spec assumes optimal conditions. Reality is about 70-80% of theoretical maximum. Labs consistently exceeding 150 daily samples should consider stepping up to modular systems.
With proper maintenance, 10-15+ years is typical. The e411 has been in production since 2006, and many original installations are still running. These are mechanically robust systems.
No. The e411 uses a closed Elecsys reagent system. Only Roche packs work. The upside is perfect result consistency and no lot-to-lot variability problems. The downside is you're tied to Roche pricing - which is why our reagent pricing matters.
Disk configuration holds 30 sample positions in a circular arrangement. Rack configuration holds 75 samples using standard 5-position racks with continuous loading capability. Both include dedicated STAT positions. Rack is better for higher volumes; disk is more compact.
The disk version needs about 4 feet of bench width (47" x 29"). The rack version is larger at 67" x 37". Both require standard power and a bacteria-free deionized water supply.
Yes, via RS232 using standard ASTM protocols. Bidirectional communication is supported. Most common laboratory information systems have existing e411 drivers.
Yes, for designated STAT assays like high-sensitivity Troponin T. The system prioritizes these samples and delivers first results in approximately 9 minutes from sample introduction. Standard assays take 18-27 minutes depending on protocol. The 9-minute cardiac STAT is the e411's standout competitive advantage.
Honestly, not much. Most failures are preventable with proper maintenance. When serious problems do occur, it's usually: measuring cell issues (replaceable), main control board failure (rare), or severe contamination from neglected maintenance (preventable). The ECL detection system is inherently robust.
No. As of 2025, Roche continues manufacturing the e411 and fully supports it with reagents, parts, and service. The platform has a 19-year production history with no announced end-of-life date.
Main analyzer unit, computer and software, sample racks/cups to begin testing, operator documentation, and 90-day to 1-year warranty depending on the unit. Installation and training available.
Daily: 5 minutes of probe cleaning and waste management. Weekly: rinse station cleaning and SysClean cycle. Annually: comprehensive PM including probes, tubing, seals, and filters. It's one of the lower-maintenance immunoassay platforms available.
Ready to bring faster immunoassay testing to your lab?

The Roche Cobas e411 delivers what small and medium labs actually need: reliable immunoassay throughput, industry-leading STAT turnaround, and results that correlate perfectly with reference laboratories. At refurbished pricing, you get Roche quality without the Roche premium.
Stop apologizing for send-out turnaround times. Stop watching cardiac patients wait for results. Stop overpaying for immunoassay capability you can afford.
Next steps:
Step 1.
Check our current e411 inventory
Step 2.
Get reagent pricing for your test volume
Step 3.
Find out what your old analyzer is worth as a trade-in
Step 4.
Start running immunoassays faster within weeks